Enlaces transversales de Book para 4.1 HIV testing services for people with presumptive and diagnosed TB
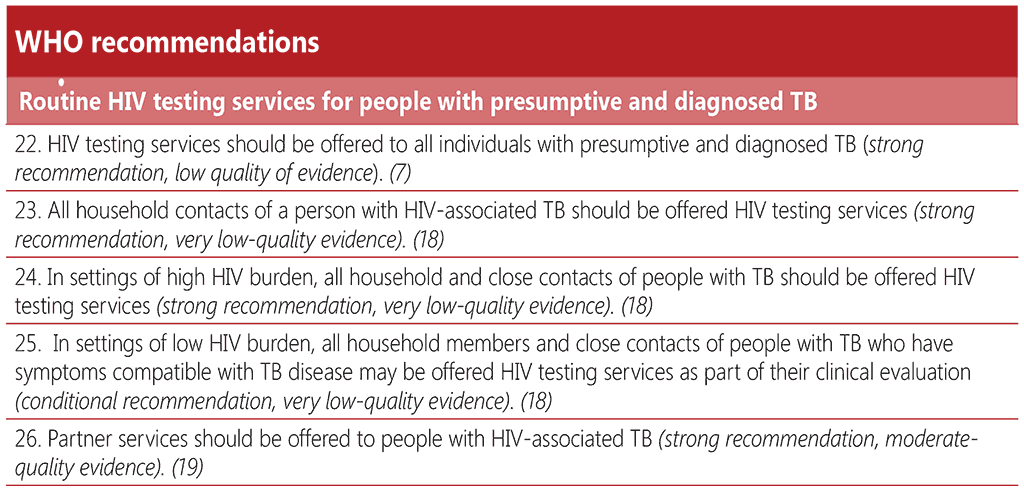
WHO recommends that all people with presumptive and diagnosed TB should be offered an HIV test as part of the essential package of TB care (17). Countries should offer HIV testing services (HTS) to 100% of people with new or relapse TB episodes, as part of the implementation of the End TB Strategy, by 2025 at the latest (133). Since the publication of the Interim policy on collaborative TB/HIV activities in 2004 (6), there has been considerable scale-up of HIV testing in people with TB, including in concentrated HIV-epidemic settings where the network of TB facilities has been leveraged to expand and decentralize HIV testing and ART services (134). However, there are still countries where there is limited coverage of HTS among people with TB, and, in many settings, offering testing services to people with presumptive TB along with household contacts of people diagnosed with TB provides a missed opportunity for targeted scale-up. Key considerations for scale-up of HTS among people with presumptive and diagnosed TB are listed in Box 4.1.
HIV testing services for people with diagnosed or presumptive TB can offer a strategic entry point for both HIV and TB prevention, care, support and treatment. HIV testing among household contacts also identifies a high number of new diagnoses of HIV infection, as the prevalence of HIV is higher than among the general adult population (7, 135). Household contacts with HIV, with unknown HIV status who are not receiving treatment, are at increased risk for rapid progression to TB disease, and at increased risk of mortality from TB.
HIV partner services is a process whereby a trained provider offers voluntary testing services to the partners and contacts of consenting HIV-positive individuals. WHO recommends a range of feasible and acceptable HIV partner service approaches to enable programmes to reach as many people with HIV as possible, which can be adapted according to setting, population, available resources and client preferences. Provider-assisted referral for HIV testing services (also called assisted partner notification, index testing or family-based index case testing) is an effective method of delivering HIV partner services to people with TB living with HIV, and is an important strategy for extending HIV testing, prevention and treatment services to their sexual partners and household members. It is also an approach for extending HIV services to drug-injecting partners. A strategic mix of facility-based, community-based, home-based and HIV self-testing (HIVST) options should be made available to ensure access to testing services across these groups.
People living with HIV who are household contacts or close contacts of someone with TB disease and who, after an appropriate clinical evaluation, are found not to have TB disease should be evaluated for TPT, even if they have previously completed a course of TPT (113). It is also important to assess eligibility for TB preventive treatment in those contacts for whom TB disease is ruled out, regardless of HIV status.
4.1.1 HIV testing service delivery approaches
WHO recommends differentiated HIV testing service delivery approaches that address the needs of a variety of population groups, geographies, contexts and epidemic settings. This includes facilitybased HTS within TB services, which is associated with improved uptake of HIV testing and HIV diagnoses among people with TB (136). There are also non-facility-based approaches to HTS, such as community-based testing, which may complement facility-based testing for people with diagnosed or presumptive TB, help address barriers to HIV testing service access, and provide an opportunity to expand HIV testing (17). Community-based HTS could be integrated as part of multidisease testing outreach and/or conducted together with TB screening as part of efforts to roll out TPT for household contacts (17). HIV testing services should never be mandatory. Policies and practices to protect vulnerable populations from mandatory or compulsory testing are needed, as well as monitoring and accountability for policies already in place. It is critical to ensure that HTS are delivered within a human rights framework. Some jurisdictions mandate HIV testing among specific groups such as immigrants. This requirement is not justified, and it can jeopardize voluntary access to health services in general and to HTS. In all circumstances, testing services should be provided in accordance with WHO’s essential 5 Cs: Consent, Confidentiality, Counselling, Correct test results and Connection (linkage to prevention, care and treatment), as outlined in Box 4.2 (137).

To enable greater access to HIV testing services, WHO also recommends lay provider-delivered HTS in healthcare facilities and in the community (137). Community health workers and lay providers such as TB champions can be trained to deliver all HTS. These include pre-test information, HIV rapid testing, interpreting HIV test results and reporting HIV status, providing post-test counselling, referrals and support to facilitate the linkage to prevention, treatment and care services.
Additional approaches and testing modalities, such as HIV partner services and HIV self-testing, can also be beneficial when delivered in facilities or in community settings. HIVST is a process in which people collect their own specimen (oral fluid or blood), perform a test using a simple rapid HIV test and interpret their results, often in a private setting, either alone or with someone they trust. Because of their flexible nature, these rapid HIV tests enable providers to offer HIV testing services wherever they are most needed, including door-to-door distribution and in different types of health facilities. A recent WHO systematic review found that in sub-Saharan Africa, offering self-testing in health facilities increased uptake of HIV testing, the number of people diagnosed with HIV and linkage to care. People with diagnosed or presumptive TB, presenting for care in private clinics or sites with limited staff and testing capacity, could benefit from accessing HIV self-test kits. Additionally, providing HIV self-test kits to people with HIV-associated TB so that they can share them with their partners and close contacts can also be a way to reach more people with HIV testing, prevention and care services.
4.1.2 Algorithms for HIV testing
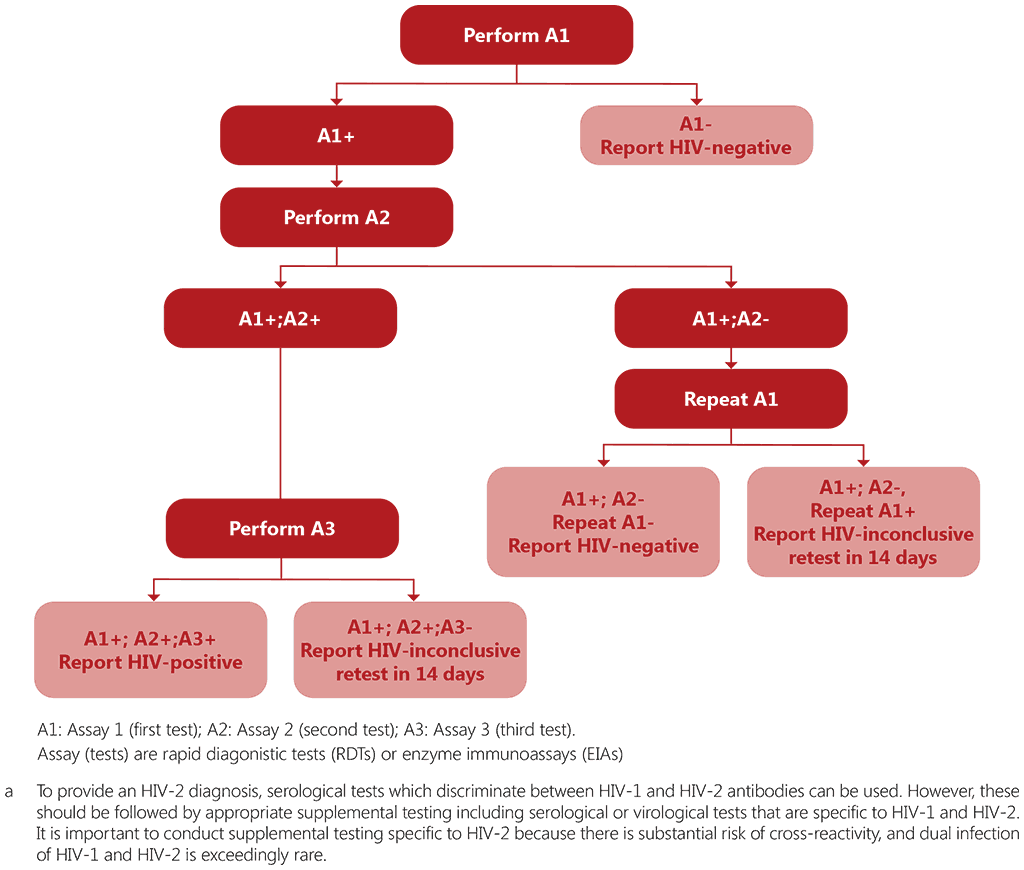
Providing a correct HIV diagnosis, as quickly as possible, is critical for all HIV testing services. Accurate diagnosis enables newly identified people living with HIV to start ART sooner, which has immediate benefits for their health and, through provider-assisted referral (index testing), for the health of their partners and the community (19). Most people testing for HIV will be negative and will likely require only one test. However, no single test can be used to provide a definitive positive HIV diagnosis. To achieve accurate results, WHO recommends that countries use a three-assay HIV testing strategy where three consecutive reactive test results are required to give an HIV-positive diagnosis (see Fig. 4.1). This strategy is considered to be the WHO standard HIV testing strategy. The HIV testing strategy can combine rapid diagnostic tests (RDTs) and/or enzyme immunoassays (EIAs) that, when used together, achieve a positive predictive value (PPV) of at least 99%. The positive predictive value indicates the probability that an HIV-positive diagnosis is correct.
The testing strategy does not stipulate the specific products to be used at each step, rather, it provides guidance on the characteristics of the test used at each step. Countries should populate the testing strategy with different products for each step, considering products that are WHO prequalified; see https://extranet.who.int/pqweb/vitro-diagnostics/vitro-diagnostics-lists. The WHO HIV testing algorithms toolkit also provides guidance to help countries optimize product selection and ensure the products selected work well together: https://www.who.int/tools/optimizing-hivtesting-algorithms-toolkit.
To achieve a PPV of at least 99%, it is critical that Assay 1 provides the best chance to rule in all HIV-positive individuals and has the highest sensitivity, while Assay 2 and Assay 3 must be able to rule out any false HIV-reactive test results, and thus should have very high specificity – higher than Assay 1.
Fig. 4.1. WHO standard testing strategy for HIV-1a diagnosis (among children or adults ≥18 months of age)
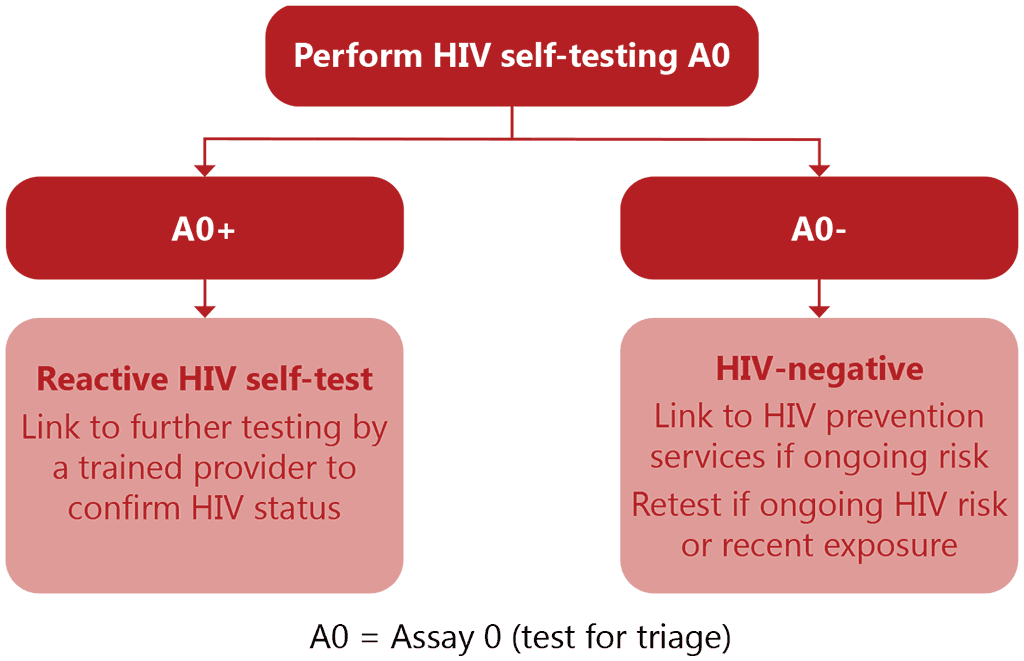
HIV self-testing can be used as a test for triage but does not provide a definitive HIV-positive diagnosis and people with reactive results need to be linked to provider-delivered testing services for HIV diagnosis. A test for triage may reduce the potential for stigma, as all individuals are tested with the same number of tests, whereas with the standard testing strategy, further testing identifies those with a reactive result on A1. Assay 0 for self-testing is not intended to replace Assay 1 of the WHO testing strategy for diagnosis. Instead, all reactive HIVST results need to be followed by further testing by a trained provider to confirm HIV status, starting with the first test in the national testing algorithm. Non-reactive self-testing results should be considered negative, with no need for further testing except for those starting pre-exposure prophylaxis (PrEP). For further details see Fig. 4.2 below and for the WHO standard HIV testing strategy and population of an HIV testing algorithm, see the WHO Consolidated guidelines on HIV testing services, 2019 (19).
Fig. 4.2. Algorithm for self-testing for HIV
4.1.3 Retesting for HIV among people with TB
Retesting for HIV is advised for people with TB for the following reasons:
- Retesting to verify a positive diagnosis before initiating lifelong ART. To ensure that individuals are not incorrectly started on lifelong ART, WHO recommends that all people newly diagnosed with HIV should be retested to verify their HIV status before starting ART, using the same testing strategy (Fig. 4.1). Misdiagnosis is difficult to detect after ART is initiated. Retesting for this purpose is a quality assessment measure that aims to detect misdiagnosis by ruling out errors related to the lot, testing site or testing provider. It may detect clerical errors, such as transcription errors during result interpretation and reporting, and specimen mix-ups. Modelling estimates indicate that retesting to identify a person incorrectly classified as HIV-positive is cost-effective and will likely cost less than unnecessary lifelong ART and virological monitoring (138).
- Retesting to provide an HIV diagnosis following inconclusive results. While most people testing for HIV will receive a diagnosis the same day, a small number of individuals will receive inconclusive results. Inconclusive results are rare but occur when (1) cross-reactivity exists between kits or patient-related factors; (2) the tester or test kit makes an error; and/or (3) individuals are seroconverting and in the window period, when infection cannot be determined. When this occurs, it is important that providers deliver messages to explain the results to clients and support them to come for retesting in 14 days. Retesting at this time will enable the provider to rule in or rule out HIV seroconversion and provide a definitive diagnosis. Retesting to resolve an HIV-inconclusive status must be conducted with the same testing strategy or algorithm and preferably at the same site.
- Retesting people with diagnosed or presumptive TB with ongoing risk of acquiring HIV, including PrEP users. While most people who test HIV-negative do not require retesting, certain population groups may need annual or more frequent retesting. In low HIV burden settings, one lifetime HIV test is sufficient for most people when there is no ongoing risk. Retesting people who are HIV-negative is only for people with ongoing HIV-related risk. In most settings, key populations require retesting at least annually. More frequent retesting every 3–6 months may be cost effective in some settings. In certain conditions and situations, individuals who have tested negative for HIV in the past can also be advised to retest. This includes, inter alia, people with presumptive or diagnosed TB and individuals with recent HIV risk exposure. Individuals from specific groups such as those receiving pre-exposure prophylaxis and pregnant women in high HIV burden settings are also encouraged to be retested. For example, people taking PrEP should receive quarterly testing, and pregnant women in high HIV burden settings should be tested at the first antenatal care visit and in the third trimester.
- Retesting to re-engage in treatment and care. In some situations, people with known HIV status may retest to check or come to terms with their diagnosis. For others, this is a way to initiate or re-engage in care. In all these situations it is important that providers offer support and deliver messages that will address concerns about their diagnosis, build trust, and encourage their successful linkage to treatment. Retesting to re-engage in treatment and care follows the same testing strategy as shown in Fig. 4.1.
4.1.4 CD4 testing
All people with HIV who enter or re-enter care should receive a CD4 cell count test at treatment baseline and as indicated for people who are clinically unstable or have advanced HIV disease (AHD)⁵ (17). CD4 cell count is the best predictor for HIV disease status, clinical staging, and immediate risk of death and should thus be used to identify those who have AHD. If viral load testing is not routinely available, CD4 count and clinical monitoring should be used to diagnose treatment failure (17). Further guidance on monitoring the response to ART and treatment failure can be found in the Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach (17).
Where possible, people living with HIV admitted to hospital or presenting to care due to serious illness should have their CD4 cell count measured. CD4 cell counts are useful to identify people who could benefit from testing for cryptococcal infection, and to help guide diagnosis and clinical management. CD4 cell counts can also help determine how likely it is that illness is due to IRIS. For people established on ART, a low CD4 cell count can be an indicator of treatment failure (which should be confirmed by viral load monitoring) and is also useful to determine whether prophylaxis for opportunistic infections is needed. Point-of-care CD4 technologies based on flow cytometry can provide results within a few minutes. Several rapid tests based on a lateral flow assay to identify patients with <200 cells/mm3 are WHO prequalified (139).
Viral load testing, where available, is indicated in any patient who is established on ART in order to identify treatment failure (17). It is recommended that routine viral load monitoring be conducted after 6 months, 12 months and then every 12 months thereafter if the person is established on ART, to synchronize with routine monitoring and evaluation reporting.
⁵ For adults and adolescent, advanced HIV disease is defined as CD4 cell count <200 cells/mm3 or WHO stage 3 or 4 event. See: Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy, July 2017 ( https://apps.who.int/iris/handle/10665/255884, accessed 31 August).
 Reacción
Reacción