Enlaces transversales de Book para 4.2.1 Area 1 – Policies, budgeting and planning
Step 1.1 – Establish a TWG, and define roles and responsibilities
A TWG comprising representatives from all key stakeholders should be established or the function of an existing TWG should be expanded to cover tests for TB infection. The TWG is critical to guide national policy development and the implementation or scale-up of TB infection testing services of the programme. The TWG should be led by the ministry of health, with a convening role for the national programmes (e.g. TB and HIV) and the national TB reference laboratory. The TWG should be mandated to advise the ministry of health, NTP and national tuberculosis reference laboratory on national policy and development of implementation tools such as testing algorithms, SOPs and protocols, and mechanisms for clinical evaluation and linkage to appropriate care and treatment. The TWG should also develop implementation plans; monitor quality of implementation and suggest steps to address gaps in implementation across the cascade of care including implementation of new tests; and review the impact and success of the programmatic introduction of testing services. Representatives from the following key stakeholders may be invited to participate:
- Ministry of health, national programmes (e.g. NTP, HIV/AIDS programmes and noncommunicable disease programmes), national TB reference laboratory and clinical (pathology) laboratory networks;
- ministry of justice and ministry of education;
- research institutes or other organizations with experience in programme implementation research and the introduction of new diagnostic tests;
- representatives from managers of municipal or provincial health offices, clinical facilities and peripheral laboratories that will participate in the programme;
- representatives from national regulatory bodies that will approve and incorporate tests and procedures;
- representatives from data management or information technology (IT) experts and managers that enable interoperability of electronic systems; and
- other technical and implementing partners outside TB and HIV programmes (e.g. community and civil society representatives), and other UN agencies
A suitably qualified individual should lead the team; for example, an officer from the NTP. An integral component of the planning process should be defining the roles and responsibilities of members of the implementation or scaling-up team, and those of external partners and donors.
Step 1.2 – Review WHO policies and available technical and implementation guides
The TWG members should familiarize themselves with the contents of the relevant WHO policies, guidance, handbooks and reports, as well as any available implementation guides from WHO, the Global Laboratory Initiative, the Foundation for Innovative New Diagnostics (FIND) and implementing partners. Particular attention should be paid to WHO policies and recommendations on who to test, how to test (using new tests) for TB infection, the limitations of tests and the interpretation of test results.
Step 1.3 – Define immediate and future purposes of tests to detect TB infection
Programmes must clearly define the purpose, scope and intended use of the new diagnostic test for TB infection, because that has Implication for planning and resource mobilization. For example, the laboratory system or network needed to provide timely results for patient-care decisions only in people who are initiating anti-TNF treatment is quite different from that needed to conduct large-scale testing for TB infection in, for example, People with HIV, household contacts of people with bacteriologically confirmed pulmonary TB, prisoners and health care workers.
Step 1.4 – Update national diagnostic algorithm and guidelines
The TWG should undertake a review of existing national policy recommendations and diagnostic algorithms, taking into consideration the needs of patients, clinical needs, country epidemiological aspects (e.g. expected number of contacts of an index patient, expected positive results for TB infection tests), existing testing algorithms, sample referral systems where IGRAs are implemented and the feasibility of different algorithms; the TWG should then make recommendations to the ministry of health and NTP. Roles of different health care personnel should be clearly established.
The TWG should advise the ministry of health and NTP on the development of clinical guidelines. Such guidelines must provide clear guidance to clinicians, pulmonologists, infectious disease doctors, nurses and health care professionals on the algorithms, target patient populations and intended use of diagnostic tests. Also, the guidelines must give special attention to new tests; how to communicate, interpret and use test results; the options for TPT regimens; and the type of contacts that should be investigated.
The TWG should be kept functional after implementation or scale-up of the TB infection programme. New evidence is constantly emerging on tests for TB infection and risk of progression, on TPT regimens , and on monitoring the quality of testing services and linkage to care and treatment according to national guidelines.
Step 1.5 – Perform a situational analysis
Recognizing the barriers in the various steps of the cascade of care and implementing specific tailored solutions for overcoming the barriers is feasible, effective, affordable and sustainable in LMIC (55–57). Digital tools have been successfully used to analyse all steps of the cascade of care and understand the main bottlenecks (58–60), and a tool to evaluate the progression on the cascade of care steps is available online (61). An example from Bastos et al. (59) is shown in Fig. 4.2. Implementation or expansion of TB infection testing using TB infection skin tests are not expected to create major barriers (15, 20). Incorporation of TBST in settings with high levels of BCG vaccination should be done with care because TBSTs are more specific than TST in such populations. In countries or settings where TST is already in place, it should be possible to implement TBST using the same systems. However, it is important to consider needs such as initial and refresher training of health care personnel in proper techniques of test administration and reading (see Areas 4 and 8), an uninterrupted supply of TB infection skin test materials and proper cold chain for transport and storage of these materials (see Area 5). Additionally, the introduction of the newer TBST may also have regulatory requirements specific to each country.
Fig. 4.2. Example of repeated cascade analyses: corrective actions taken before and after implementation, to improve retention in the TB infection cascade of care (from Brazil)
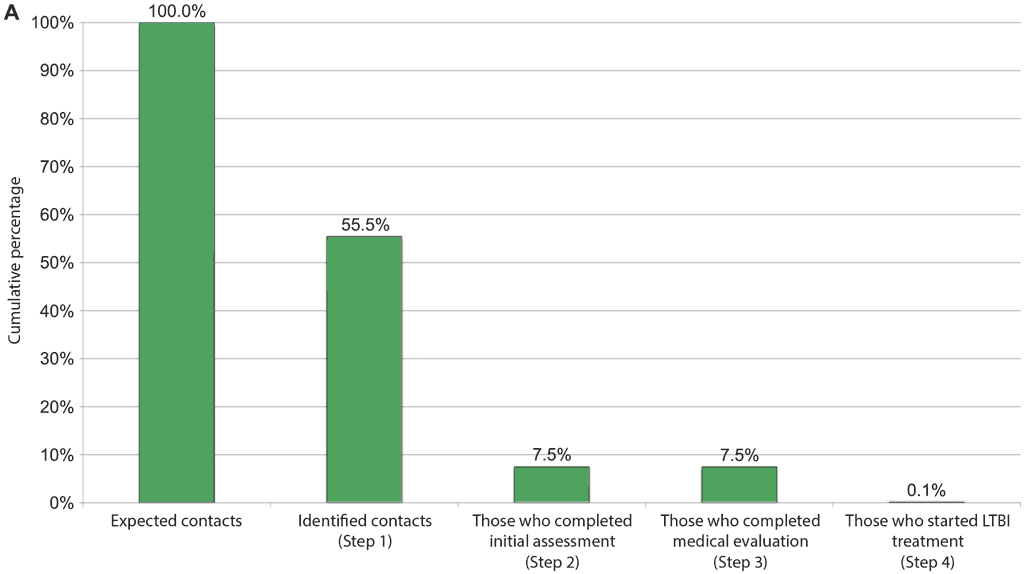
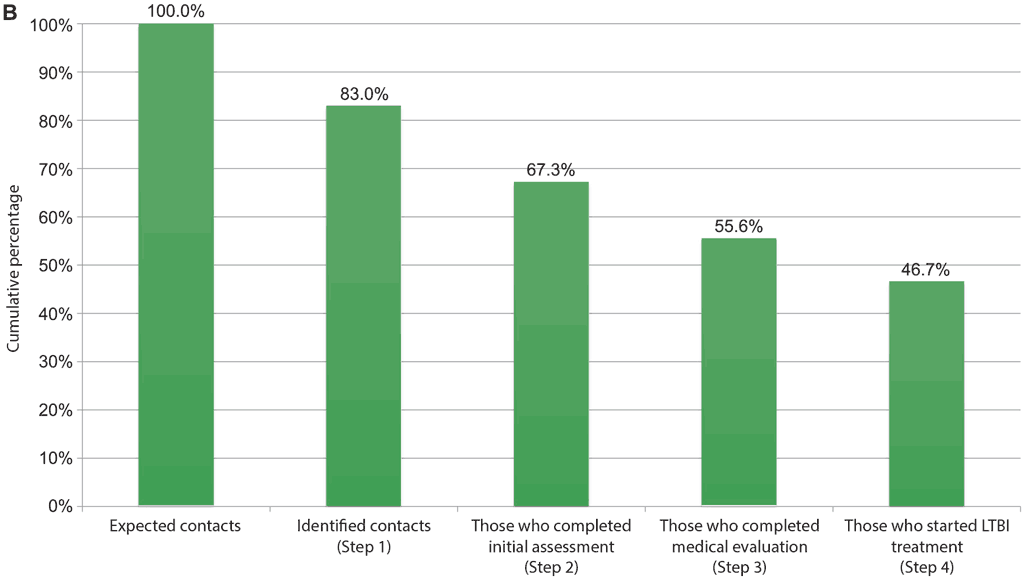
TB: tuberculosis; LTBI: latent tuberculosis infection.
In this example, solutions implemented to improve the cascade of care included sensitization and training of health care providers in primary care clinics and distribution of educational materials to health care professionals and patients.
A) Cumulative percentage of contacts of all ages retained at each step in the care cascade, streamlined evaluation phase;
B) Cumulative percentage of contacts of all ages retained at each step in the care cascade, May–October 2018.
Source: Bastos et al. (2020) (59).
Implementation of IGRAs requires laboratory and network infrastructure, a functioning sample transportation system, IT capabilities, diagnostics connectivity, and availability and adequacy of SOPs. An example of a checklist for evaluating a specimen referral system is available (62). Staff skills, expertise and experience should be reviewed and updated if needed (see Areas 4 and 8).
The situational assessment should also include identification of gaps in systematic recording and reporting of events across the cascade of care for TB screening and TPT. The TWG should also advise on data variables to be included in the national HMIS system, to enable quality implementation and monitoring and evaluation of services for testing for TB infection.
Step 1.6 – Develop a costed operational plan for phased implementation or scale-up
The final step in Area 1 is to develop a detailed, costed and prioritized action plan for phased programmatic implementation, with coverage targets and a timeline. Often, implementation of a new interventions must overcome potential challenges such as the cost of human resources, cost of instruments, ancillary equipment and consumables; requirements for improving or establishing the necessary laboratory and network infrastructure (e.g. a specimen transport system); the need for specialized, skilled and well-trained staff; the need for expert technical assistance; maintenance of confidentiality of patient information; and establishment of a quality assessment system (see Area 8).
Staff time: Using time and motion methodology, TST was found to be the least time-consuming step in the TB infection cascade of care. In LMIC, an average of 3.1 minutes was required for TST application, and 3.2 minutes for TST reading (63). Labour time for ELISA-based IGRAs is estimated by the manufacturer to be less than 1 hour per ELISA plate; however, because of the need to incubate the test for at least 16 hours, the entire laboratory process usually takes about 24 hours (55). T-SPOT.TB needs more personnel hours because this test requires PBMCs to be separated and counted initially, although results are available within 24 hours for this test as well. Given that personnel costs vary according to the setting, total costs for human resources should be estimated in each country.
Costs of tests: When estimating costs of the test, apportionment of common equipment and the laboratory area used for other tests should be estimated according to the workflow in each laboratory. This can vary widely by setting. Successful implementation of the plan will require financial and human resource commitments from the ministry of health or NTP, with possible support from implementing partners. A budget should be developed to address activities in collaboration with key partners. For skin testing, investments to maintain a cold chain for the testing material can be considered in collaboration with the national immunization programme. Additional investments to enhance capacities for a cold chain may be factored in if necessary.
 Reacción
Reacción